DEDICATED
to advancing THB335 for the treatment of multiple mast cell-mediated inflammatory diseases
We’re advancing our lead product candidate, THB335, for the treatment of multiple mast cell-driven inflammatory diseases of the skin, airway and gastrointestinal tract.
THB335 is a potent, highly selective, oral small molecule KIT inhibitor. Our initial development focus for THB335 is in chronic spontaneous urticaria. We plan to pursue the development of THB335 in additional diseases, including severe asthma, where mast cells play a known role in their pathophysiology and symptomatology.
About
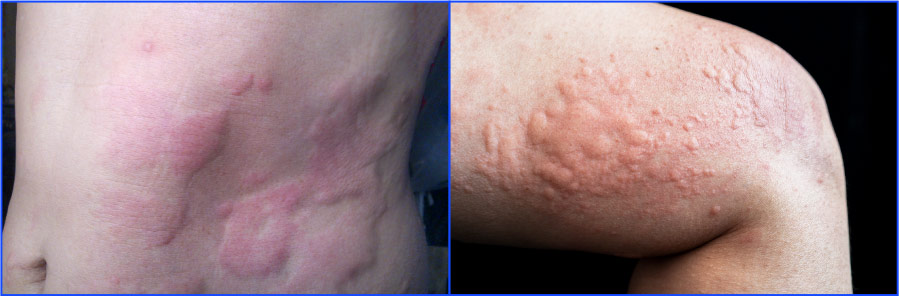
Chronic urticaria is a dermatologic condition driven by mast cell activation that results in red, itchy, painful welts or hives that can be accompanied by deep tissue swelling, burning, stinging, numbness, and tingling. The condition carries a significant burden for patients, causing physical discomfort and emotional distress, including anxiety, depression, impaired social and work function, sleep disruption, and social isolation.
Up to 1% of the US population is believed to be affected by urticaria at some point in their lives. Standard antihistamines, the first line of therapy, are only effective in about half of patients. An injectable biologic therapy is the only approved second-line treatment and provides complete symptom relief in a minority of patients.
New treatment options with the potential to achieve robust disease control are imperative to driving awareness, diagnosis and treatment of CSU.

About
Asthma
treatment options
Severe asthma is a chronic inflammatory condition marked by reduced lung function and higher incidence of potentially life-threatening exacerbations despite use of standard nonbiologic therapies. Mast cells have been shown to play a central role in the regulation of both lower airway inflammation and airway hyperresponsiveness, two key elements of asthma pathophysiology. The severe asthma population represents 5-10% of all people living with asthma. Despite the availability of multiple injectable biologics for treatment of severe asthma, effective oral treatment options are severely limited.
We plan to develop THB335 in severe asthma.

Trials
THB335 is currently being evaluated in a Phase 1 clinical trial to evaluate the safety, pharmacokinetic and pharmacodynamic profile of THB335.
Learn more about the trial